
THE FIRST TREATMENT SPECIFICALLY
approved for adult patients with
KRAS-mutated recurrent LGSOC
To learn more, explore the helpful resources below
AVMAPKI FAKZYNJA CO-PACK is indicated for the treatment of adult patients with KRAS-mutated recurrent low-grade serous ovarian cancer (LGSOC) who have received prior systemic therapy.
This indication is approved under accelerated approval based on tumor response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial.
For additional information, please call 833-MED-VSTM (833-633-8786)
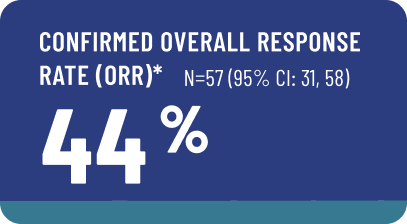

The efficacy of AVMAPKI FAKZYNJA CO-PACK was evaluated in RAMP-201, an open-label, multicenter study that included 57 adult patients with measurable KRAS-mutated recurrent LGSOC.
- Patients were required to have received at least one prior systemic therapy, including a platinum-based regimen.
- Median age of patients in the study was 60 years (range 29 to 87 years).
- 75% were White, 3.5% were Asian, 3.5% were Black or African American, and 18% did not have race reported; 3.5% of patients were Hispanic or Latino.
- Fourteen percent of patients had received 1 prior line of systemic therapy, 25% of patients had received 2 prior lines, 18% had received 3 prior lines and 40% had received more than 3 prior lines. All patients had received prior platinum-based chemotherapy.
- 84% received prior hormonal therapy (as maintenance or treatment), 40% received prior bevacizumab and 21% received a prior MEK inhibitor.
- Patients received AVMAPKI 3.2 mg orally twice weekly for the first 3 weeks out of a 4-week cycle and FAKZYNJA 200 mg orally twice daily for the first 3 weeks out of a 4-week cycle until disease progression or unacceptable toxicity.
- Patients were excluded if they were candidates for debulking surgery, were on treatment with warfarin, had an active skin disorder requiring systemic therapy within the past year, or had an ocular disorder (including a history of retinal pathology, an active or chronic visually significant corneal disorder, or a history of glaucoma).
Please read the Important Safety Information.
AVMAPKI FAKZYNJA CO-PACK Resources

Verastem CaresTM is designed to support your efforts to address patient access challenges. We are available to assist with insurance navigation, financial and patient assistance programs, logistics support and educational resources.
For more information and helpful resources, call Verastem CaresTM at




